Specialist Dental Group has launched an on-going series of blog posts by our individual dental specialists. All views provided are the dentist’s own opinions and are posted on this blog as part of our on-going efforts to educate the public about dental issues and other matters of interest relating to dentistry and healthcare.
We are all aware that regular tooth-brushing and flossing will prevent tooth decay and gum disease. Diet choices also play a part: consuming less sweet snacks and drinks is also advisable.
However, not many people may be aware of a different sort of tooth destruction that takes place even in young, healthy persons.
What is your preferred drink on a hot day? Carbonated “fizzy” drinks? What about orange juice or lime juice?
What do you like to consume after a session of sports? Sports drinks that are said to replenish minerals lost through perspiration?
These drinks have a high level of acid – in fizzy drinks, this happens through the process of pumping carbon dioxide into the drink under pressure; in fruit juices, the acid is naturally occurring. The pH of such drinks range from 2.5 to 3.6. At such levels, the acid can cause erosion to your enamel, the hard, protective outer layer of our teeth. Dissolution of enamel already begins at a higher pH level of 5.5. When enamel is eroded away, tooth sensitivity will occur.
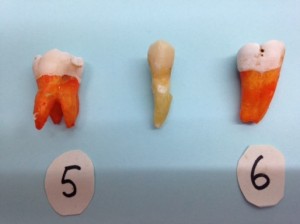
Recently, I was carrying out a school project with my son. We collected teeth that had no decay or fillings (teeth that were extracted due to gum disease). After the teeth were cleaned, they were soaked in a carbonated beverage, a sports drink, orange juice and water, respectively for 3 weeks.
Using a highly sensitive weighing scale (capable of measuring up to 3 decimal places in grams), the teeth were weighed at the start, then again at the end of each week for 3 weeks after soaking in the above-mentioned beverages.
Guess what? There was a noticeable loss of mass for each and every tooth ranging from 0.05 to 0.1grams. This is 4-8% loss of tooth mass just in three weeks, imagine the detrimental effect in the long term. That’s a significant result! The enamel of all the teeth (except the one soaked in water) also showed marked frostiness (see Figure 1 above). This white, frosted appearance is known to dentists as a sign of demineralisation, meaning that the organic and some of the hydroxyapatite crystal structure in enamel is dissolved away.
In the next step of the experiment, we used a fingernail file to scrape against the enamel. Fine, white powder was easily scraped off the teeth. One particular tooth actually broke into 2 pieces! (see Figure 2 on the left)
Can you imagine your teeth literally being dissolved away by such drinks? Of course it may be argued that saliva provides protection against the onslaught of acid. Then we swallow our drinks quickly; we seldom hold them for long in the mouth. However, we have used the above experiment to demonstrate how detrimental the long-term, regular consumption of acidic, carbonated drinks can be.
Furthermore, tooth erosion occurs on the smooth surfaces of teeth, not in the pits and grooves as with normal tooth decay. Tooth erosion is spread over a wider surface area and can be very difficult to restore with the traditional filling materials. So be aware of what you and your loved ones are drinking on a regular basis. Don’t let your teeth “melt” away.

Dr Helena Lee is a Dental Specialist in Periodontics with Specialist Dental Group®, Singapore. She is also an Adjunct Lecturer with the National University of Singapore. Dr Lee has a special interest in dental implants, gingival plastic surgery, and tissue grafting. For more information, visit www.specialistdentalgroup.com